1.2 Scientific method
|
Previous
1.1 The development of a scientific theory
|
Next
1.3 Data and data analysis
|
1.2 Scientific method (ESCHT)
The scientific method is the basic skill process in the world of science. Since the beginning of time humans have been curious as to why and how things happen in the world around us. The scientific method provides scientists with a well structured scientific platform to help find the answers to their questions. Using the scientific method there is no limit as to what we can investigate. The scientific method can be summarised as follows:
-
Ask a question about the world around you.
-
Do background research on your questions.
-
Make a hypothesis about the event that gives a sensible result. You must be able to test your hypothesis through experiment.
-
Design an experiment to test the hypothesis. These methods must be repeatable and follow a logical approach.
-
Collect data accurately and interpret the data.You must be able to take measurements, collect information, and present your data in a useful format (drawings, explanations, tables and graphs).
-
Draw conclusions from the results of the experiment. Your observations must be made objectively, never force the data to fit your hypothesis.
-
Decide whether your hypothesis explains the data collected accurately.
-
If the data fits your hypothesis, verify your results by repeating the experiment or getting someone else to repeat the experiment.
-
If your data does not fit your hypothesis perform more background research and make a new hypothesis.
Remember that in the development of both the gravitational theory and thermodynamics, scientists expanded on information from their predecessors or peers when developing their own theories. It is therefore very important to communicate findings to the public in the form of scientific publications, at conferences, in articles or TV or radio programmes. It is important to present your experimental data in a specific format, so that others can read your work, understand it, and repeat the experiment.
-
Aim: A brief sentence describing the purpose of the experiment.
-
Apparatus: A list of the apparatus.
-
Method: A list of the steps followed to carry out the experiment.
-
Results: Tables, graphs and observations about the experiment.
-
Discussion: What your results mean.
-
Conclusion: A brief sentence concluding whether or not the aim was met.
A hypothesis
A hypothesis should be specific and should relate directly to the question you are asking. For example if your question about the world was, why do rainbows form, your hypothesis could be: Rainbows form because of light shining through water droplets. After formulating a hypothesis, it needs to be tested through experiment. An incorrect prediction does not mean that you have failed. It means that the experiment has brought some new facts to light that you might not have thought of before.
In science we never 'prove' a hypothesis through a single experiment because there is a chance that you made an error somewhere along the way. What you can say is that your results SUPPORT the original hypothesis.
In the analysis of the scientific method activity:
-
The question may already be answered in the literature, or there may be background research that you can build upon. It is also best to make a hypothesis, prediction and an experiment with as good an understanding of the topic as possible.
-
A controlled variable is one which you keep constant (controlled) so that it does not have an effect between readings. The independent variable is the one you change between collecting data points, while the dependent variable is the variable that changes as a result of a change in the independent variable.
-
It is important to identify all the variables that you think will have an effect on your investigation.
-
Firstly think of all the relevant variables you can change.
-
Secondly think of all the variables you can measure or observe.
-
Thirdly choose one variable to change (independent variable) which will have an effect on the one variable you can measure or observe (dependent variable).
-
All the other variables you need to keep constant (fixed/controlled variable).
-
-
-
Identifying a problem involves thinking about the world around you and a specific part of it that you don't understand.
-
A hypothesis is more formal, it is a prediction about that problem based on your current understanding and background research.
-
A scientific theory comes from an experimentally tested and proven hypothesis. It is repeatable and current data fits the theory.
-
-
Data may fit a hypothesis in a specific instance. That does not mean that the hypothesis is generally true. It is important to repeat the experiment to make sure that the experiment was not an anomaly. Before something becomes a scientific theory it must be tested repeatedly and be repeatable by different people.
Analysis of the scientific method.
Break into groups of 3 or 4 and study the flow diagram provided, then discuss the questions that follow.
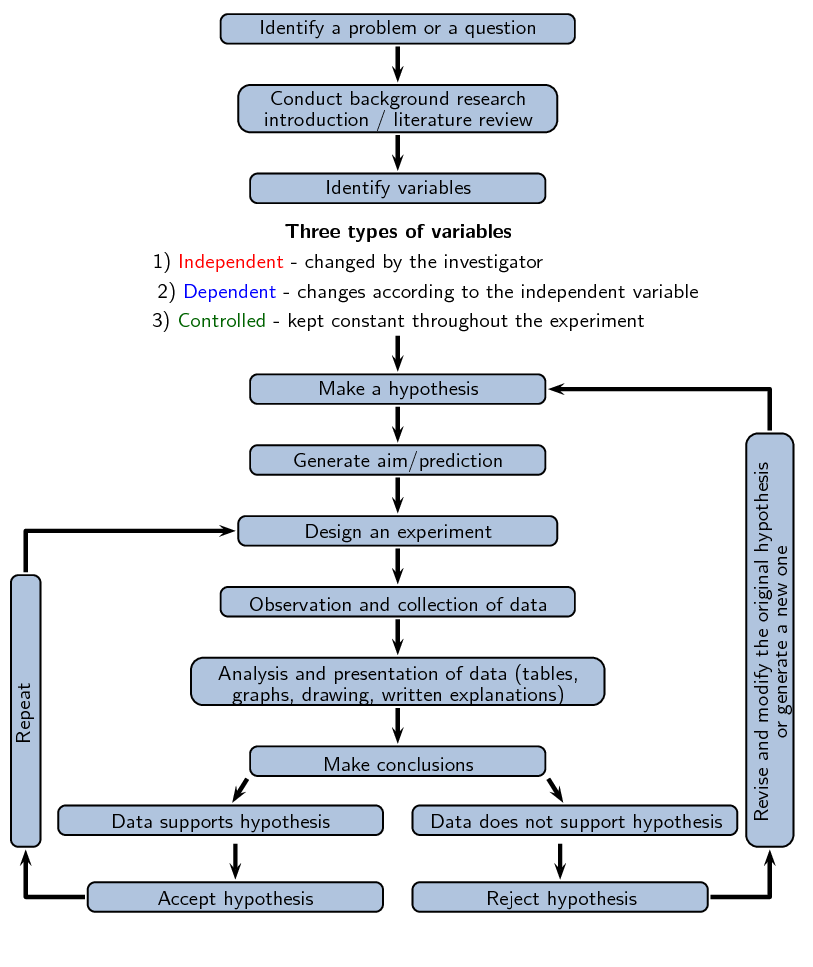
Figure 1.2: Overview of scientific method.
-
Once you have a problem you would like to study, why is it important to conduct background research before doing anything else?
-
What is the difference between a dependent, independent, and controlled variable and why is it important to identify them?
-
What is the difference between identifying a problem, a hypothesis, and a scientific theory?
-
Why is it important to repeat your experiment if the data fits the hypothesis?
When the learners design their own experiment:
-
An example of a question that the learner might ask would be why do rainbows form?
-
Before beginning an investigation background research needs to be undertaken. Background research should always be referenced.
In this example the type of background research might include the particles found in the atmosphere, the diffraction of light through water and the different wavelengths of light.
-
Learners must write down a statement that answers their question. This is the hypothesis and should be specific, relating directly to the question they are asking.
In this example their hypothesis might be: Rainbows form because of the diffraction of light through water droplets in the atmosphere. If light is shone through water at the right angle a rainbow will form. Their hypothesis should be testable.
-
The learners should identify variables that are important in their specific experiment. For example, the temperature of the water, the type of light they use, the purity of the water, the angle of the light could all be variables in their experiment.
-
The learners should understand the difference between independent, dependent and controlled variables and be able to identify them in their experiment.
For example, the type of light might be controlled (sunlight). The temperature could be a independent variable (perform the experiment on different days with different temperatures). With the temperature as an independent variable then the angle the light comes out at could be a dependent variable, to see if there is a link. If the temperature is made a controlled variable on that day, then the angle could be the independent variable, and the shape and size of the rainbow would be the dependent variable.
-
The learner must design an experiment that accurately tests their hypothesis. The experiment is the most important part of the scientific method. These are all important concepts to know when designing an experiment:
-
The method should be written so that a complete stranger will be able to carry out the same procedure in the exact same way and get almost identical results.
-
The method must be clear and precise instructions including the labelling of apparatus, giving exact measurements or quantities of chemicals or substances to be used and making sure that all the apparatus used is listed.
-
The method must give clear instructions about/describing how the results should be recorded (table, graph, etc.)
-
The method should include safety precautions where possible.
-
-
The learner should present a one page experimental write up with an aim, the apparatus necessary, the method that will be followed. An example experiment is given here. Note that this is just an example, the learners could perform their experiment on anything.
-
Aim
The aim of this experiment is to determine what happens when sunlight is shone through a glass of water.
-
Apparatus
-
A clear \(\text{500}\) \(\text{ml}\) glass, three A4 pieces of white paper, a tape measure, sticky tape, a pencil, a thermometer
-
At least \(\text{400}\) \(\text{ml}\) of water, sunlight.
-
-
Method
-
Use the sticky tape to stick an A4 piece of a paper to a sunny wall exactly \(\text{1}\) \(\text{m}\) from the ground.
-
Use the sticky tape to stick an A4 piece of paper above the first one, and the last A4 piece of paper below the first one.
-
Fill the glass with \(\text{400}\) \(\text{ml}\) water and measure the temperature (be careful to keep the thermometer out of direct sunlight between measuring the temperatures.
-
Hold the glass (near the bottom) level with the bottom of the middle piece of paper in the sunlight. If a rainbow forms on the sheets of paper, mark where it forms on the paper.
-
Measure the temperature of the water in the glass, then move the glass upwards \(\text{5}\) \(\text{cm}\) and repeat.
-
Repeat step \(\text{5}\) until you reach the top of the uppermost A4 piece of paper.
-
-
Results
-
Your results should be presented in the form of a table (Result? should be answered with a yes or a no depending on whether a rainbow formed):
Temperature (\(^{\circ}\)C)
Height of glass (m)
Result?
Height of rainbow
From this data the angle of refraction of the water can be measured, as well as what angle is required for the sunlight to create a rainbow through the water.
-
-
Designing your own experiment
Recording and writing up an investigation is an integral part of the scientific method. In this activity you are required to design your own experiment. Use the information provided below, and the flow diagram in the previous experiment to help you design your experiment.
The experiment should be handed in as a \(\text{1}\) - \(\text{2}\) page report. Below are basic steps to follow when designing your own experiment.
-
Ask a question which you want to find an answer to.
-
Perform background research on your topic of choice.
-
Write down your hypothesis.
-
Identify variables important to your investigation: those that are relevant, those you can measure or observe.
-
Decide on the independent and dependent variables in your experiment, and those variables that must be kept constant.
-
Design the experiment you will use to test your hypothesis:
- State the aim of the experiment.
- List the apparatus (equipment) you will need to perform the experiment.
-
Write the method that will be used to test your hypothesis
- in bullet format
- in the correct sequence, with each step of the experiment numbered.
- Indicate how the results should be presented, and what data is required.
Reading instruments (ESCHV)
Before you perform an experiment you should be comfortable with certain apparatus that you will be using. The following pages give some commonly used apparatus and how to use them.
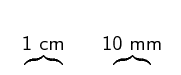

Figure 1.3: The end of a ruler.
Most rulers you find have two sets of lines on them. You can ignore those with the numbers spaced further apart. We only work in the metric system and those are for the imperial system. The closest together lines are for millimetres, the thicker lines are for \(\text{5}\) \(\text{mm}\) and the thicker, longer lines with numbers next to them mark off every \(\text{10}\) \(\text{mm}\) (\(\text{1}\) \(\text{cm}\)).

Figure 1.4: Reading a thermometer.
A thermometer can have one, or two sets of numbers on it. If it has two sets of numbers one will be in Celsius, and one will be in Fahrenheit. We use Celsius, so you can ignore the side with a larger temperature range. In Figure 1.4 you can ignore the right-hand side. Looking on the left you can see that the red line (coloured ethanol here) is next to the fourth line above \(\text{0}\) \(\text{℃}\). Each small line is \(\text{1}\) \(\text{℃}\), so the temperature is \(\text{4}\) \(\text{℃}\).

Figure 1.5: A laboratory style thermometer.
Laboratory thermometers will go to much higher temperatures than those used for measuring the temperature outside, or your body temperature. It is important to make sure that the thermometer you are using can handle the temperature you will be measuring too. If not, do not use that thermometer as you will break it. Make sure your thermometer is upright whenever you use it in an experiment, to avoid incorrect results.

Figure 1.6: A scale (also referred to as a balance).
Different scales have different functions. However, a basic function of all scales is a tare button. This zeros the balance. It is important that you zero the balance before you take any measurements. If you are weighing something on a piece of paper you should tare the balance with the piece of paper on it, and weigh the substance. Make sure you check the units that your scale is weighing in. If you want your value to be accurate to \(\text{,00}\) \(\text{g}\) then the scale must measure to that accuracy. A scale that measures in \(\text{mg}\) would be best.
A burette is used to accurately measure the volume of a liquid added in an experiment. The valve at the bottom allows the liquid to be added drop-by-drop, and the initial and final volume can be measured so that the total volume added is known. More information about burettes is given to you in your first titration experiment this year in Chapter 9.
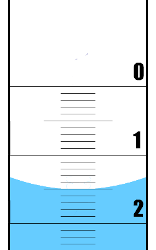
Figure 1.7: The meniscus of water in a burette.
The surface of the water (the meniscus) is slightly higher at the edges of a container than in the middle. This is due to surface tension and the interaction between the water and the edge of the container (Figure 1.7). When measuring the volume in a burette (or measuring cylinder or pipette) you should look at the bottom of the meniscus. Where that lies is where you measure the volume. So in this example the meniscus is on the fifth line below the large line that represents \(\text{1}\) \(\text{ml}\). Therefore the volume is \(\text{1,5}\) \(\text{ml}\).
It is also possible that the liquid being measured has greater internal forces than those between it and the container. Then the meniscus would be higher in the middle than at the sides, and you would use the top of the meniscus to measure your volume.

Figure 1.8: A measuring cylinder with water.
A measuring cylinder is used to measure volumes that you want accurate to the nearest millilitre or so. It is not a highly accurate way of measuring volumes. The volume in a measuring cylinder is measured in the same way as for a burette, the difference is that in a measuring cylinder the smallest volume would be at the bottom, while the largest would be at the top.

Figure 1.9: A \(\text{20}\) \(\text{ml}\) volumetric pipette.

Figure 1.10: A graduated pipette.
There are two types of pipettes you might encounter this year. A volumetric pipette has a large bulb, marked with the set volume it can measure. Above the bulb on these pipettes there is a line. For a \(\text{5}\) \(\text{ml}\) volumetric pipette, when the meniscus of your liquid sits on the line, then the volume in that pipette is \(\text{5}\) \(\text{ml}\).
A graduated pipette has the same type of marking you see on a burette. The top is \(\text{0}\) \(\text{ml}\), and the volume increases as you move down the pipette. In this pipette you should fill the pipette to near the \(\text{0}\) \(\text{ml}\) line and make a note of the volume. You can then add the desired volume, stopping when the volume in the pipette has decreased by the required amount.
Performing experiments (ESCHW)
A learner wondered whether the rate of evaporation of a substance was related to the boiling point of the substance. Having done background research they realised that the boiling point of a substance is linked to the intermolecular forces within the substance. They know that greater intermolecular forces require more energy to overcome. This led them to form the following hypothesis:
The larger the intermolecular forces of a substance the higher the boiling point. Therefore, if a substance has higher boiling point it will have a slower rate of evaporation.
Perform the following experiment that the learner designed to test that hypothesis.
The boiling points and rate of evaporation experiment is a very simple one meant to introduce the learners to the concept of the scientific method in a practical way. It has been broken up into three parts: performing the practical investigation, analysis of results, drawing conclusions.
The learners should be as accurated as possible when measuring the drop in volume as they will be required to plot a graph of their data.
Boiling points and rate of evaporation: Part 1
Aim
To determine whether the rate of evaporation of a substance is related to its boiling point.
Apparatus
You will need the following items for this experiment:
-
\(\text{220}\) \(\text{ml}\) water, \(\text{20}\) \(\text{ml}\) methylated spirits, \(\text{20}\) \(\text{ml}\) nail polish remover, \(\text{20}\) \(\text{ml}\) water, \(\text{20}\) \(\text{ml}\) ethanol
-
One \(\text{250}\) \(\text{ml}\) beaker, four \(\text{20}\) \(\text{ml}\) beakers, a thermometer, a stopwatch or clock
Method
All alcohols are toxic, methanol is particularly toxic and can cause blindness, coma or death. Handle all chemicals with care.
-
Place \(\text{200}\) \(\text{ml}\) of water into the \(\text{250}\) \(\text{ml}\) beaker and move the beaker to sunny spot. Place the thermometer in the water.
-
Label the four \(\text{20}\) \(\text{ml}\) beakers \(\text{1}\) - \(\text{4}\). These beakers should be marked.
-
Place \(\text{20}\) \(\text{ml}\) methylated spirits into beaker 1, \(\text{20}\) \(\text{ml}\) nail polish remover into beaker 2, \(\text{20}\) \(\text{ml}\) water into beaker 3 and \(\text{20}\) \(\text{ml}\) ethanol into beaker 4.
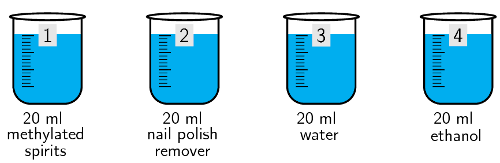
-
Carefully move each beaker to the warm (sunny) spot.
-
Observe each dish every two minutes. Note the volume in the beaker each time.
-
Continue making observations for \(\text{20}\) \(\text{minutes}\). Record the volumes in a table.
Results
-
Record your observations from doing the investigation in a table like the one below.
Substance
Methylated
spirits
Nail polish
remover
Water
Ethanol
Boiling point (\(^{\circ}\)C)
\(\text{78,5}\)
\(\text{56,5}\)
\(\text{100}\)
\(\text{78,4}\)
Initial volume (ml)
\(\text{20}\)
\(\text{20}\)
\(\text{20}\)
\(\text{20}\)
\(\text{2}\) \(\text{min}\)
\(\text{4}\) \(\text{min}\)
\(\text{6}\) \(\text{min}\)
|
Previous
1.1 The development of a scientific theory
|
Table of Contents |
Next
1.3 Data and data analysis
|
